ENDONEXT™
BETTER FOR YOUR LAB. BETTER FOR THE PLANET.
ENDONEXT™ endotoxin detection assays are ushering in a new era of smarter, more sustainable pharmaceutical quality control.
- ENDOLISA Endotoxin testing
- Features
- Tests
- Downloads
- Videos
Product Details
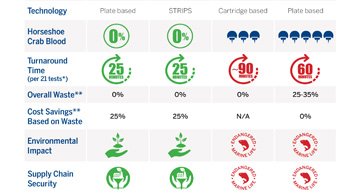
Based on recombinant Factor C (rFC), ENDONEXT™ technology not only eliminates the need to harvest horseshoe crab blood, but also makes your laboratory more efficient. With 100% endotoxin specificity, lot-to-lot consistency, and more streamlined workflows, ENDONEXT™ provides reliable results everywhere from in-process controls to final product testing on the most complex matrices.
Features
- Easy automation with robotic liquid-handling systems
- Fluorescence end-point assays in a 96-well microplate format
Main Benefits
100% endotoxin specificity
High lot-to-lot consistency
Ecologically sustainable
Validated according to standard pharmacopoeia Bacteria Endotoxin Testing criteria
Tests
We make endotoxin testing FASTER, EASIER, and ENVIRONMENTALLY FRIENDLY, plus lot-to-lot consistency gives you confidence in your in-process control and product release decisions. Explore the ENDONEXT™ range of products.
ENDOZYME® II
The enhanced second generation of ENDOZYME® is a flexible and easy-to-use endotoxin detection assay.
- State-of-the-art sensitivity down to 0.001 EU/mL
- Flexible assay time depending on required sensitivity
- Particularly suited for final product testing, formulation and research
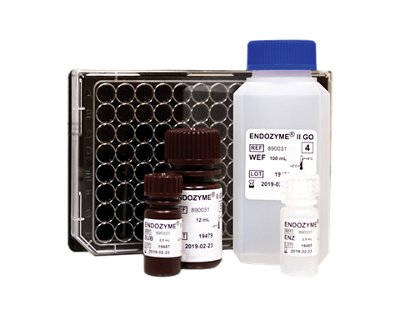
ENDOZYME® II GO
The rapid GO version of ENDOZYME®II featuring GOPLATE™ - a microplate pre-filled with required standard curve and positive product control concentrations (PPC).
- Eliminates manual preparation of standard dilutions and PPCs
- Over 50% reduction in handling time compared to conventional microplate assays
- Significantly reduces risk of error
- Ideal for in-process control of water and raw materials as well as product release testing
- Easy automation
ENDOZYME® II GO STRIPS
ENDOZYME® II GO STRIPS contain all of the benefits of ENDOZYME® II GO with simple and consistent use due to the CSE pre-coated wells, with the flexibility to test anywhere from 1 to 21 samples with minimal waste.
Laboratories do not always have 20 samples to fill a full plate; ENDOZYME® II GO STRIPS are designed with flexibility in mind— flexible number of samples and flexible testing timing.
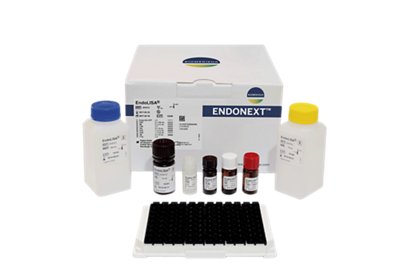
ENDOLISA®
With its unique built-in sample preparation step, the ENDOLISA® assay revolutionizes endotoxin testing of complex samples.
- ELISA-like format features 96-well plate pre-coated with a specific endotoxin-binding phage protein
- Overcomes limits of traditional methods such as inhibition and enhancement
- Unprecedented tolerance of organic solvents, detergents
Downloads
Brochure - ENDONEXT™ - The Evolution of Endotoxin Testing
Download
- Filename
- PHARMA_BROCHURE_ENDONEXT_A4_05_23_9324113_Pages.pdf
- Size
- 488 KB
- Format
- application/pdf
Brochure - ENDONEXT™ Software - Your new Lab Assistant Efficient and 21 CFR Part 11 compliant
Download
- Filename
- BROCHURE_ENDONEXT_SOFTWARE_A4_05_23_9324112.pdf
- Size
- 277 KB
- Format
- application/pdf
Brochure - ENDOZYME® II - Recombinant Factor C (rFC) Endotoxin Detection Assay
Download
- Filename
- Flyer_ENDOZYME II.pdf
- Size
- 867 KB
- Format
- application/pdf
Brochure - ENDOZYME® II GO - Endotoxin Testing Made Faster, Easier, and Sustainable. Ally Friendly.
Download
- Filename
- PHARMA_BROCHURE_EZIIGO_A4_05_23_9324100_Pages.pdf
- Size
- 574 KB
- Format
- application/pdf
Brochure - ENDOZYME® II GO Strips - Do you need flexible Endoxotin testing?
Download
- Filename
- PHARMA_BROCHURE_EZIIGO_STRIPS_A4_05_23_9324100_Pages2.pdf
- Size
- 483 KB
- Format
- application/pdf
Brochure - ENDOLISA® - Endotoxin Detection Assay Based on ELISA-Technology and Recombinant Factor C (rFC)
Download
- Filename
- Flyer_ENDOLISA.pdf
- Size
- 920 KB
- Format
- application/pdf
Citation List - Recombinant Factor C (rFC) Assay
Download
- Filename
- BMX_rFC_Citation_List_2010_2022_Final.pdf
- Size
- 74 KB
- Format
- application/pdf
ENDONEXT™ The Evolution Of Endotoxin Testing
Eliminates the need to harvest horseshoe crab blood and helps make quality control more precise and efficient.